This is post #6 of this series on the Brain (First Post and Last Post). As I mention every time, these posts are from a series of lectures by Prof. Jan Schnupp, and I want to make sure he is properly quoted and credited. Many parts are his lectures verbatim. However, for any errors, mistakes or inaccuracies in anything I write in here, I take all the credit. I probably misunderstood him or made it up. His course material is available here.
We are going to be talking about synapses in this post, specifically chemical synapses. Now, last time we said that the brain is an electrical organ. It is electrical in some sense. But it is also a chemical organ. It is just as chemical as it is electrical.
Neurons form a network
As a quick recap of what we talked about last time: A neuron carries electrical potentials across its membrane, so their membranes are electrically charged, and there are little ion channels in the membrane that can open and close. Various factors, including environmental stimuli, can cause these channels to open and close, allowing ions to flow in, which changes the membrane voltage. This is how the brain encodes information from the outside world and processes signals internally.
Now, neurons make poor cables. To set up their voltages across their membranes, they allow potassium ions to leak out, which makes them inefficient conductors. Therefore, they must constantly renew currents to produce nerve impulses, or spikes, that can travel over longer distances. Of course, a single neuron can't do much on its own; we have about a hundred billion neurons, which tells us they work best in large networks. But so far, there's nothing to explain how signals move from one neuron to the next.
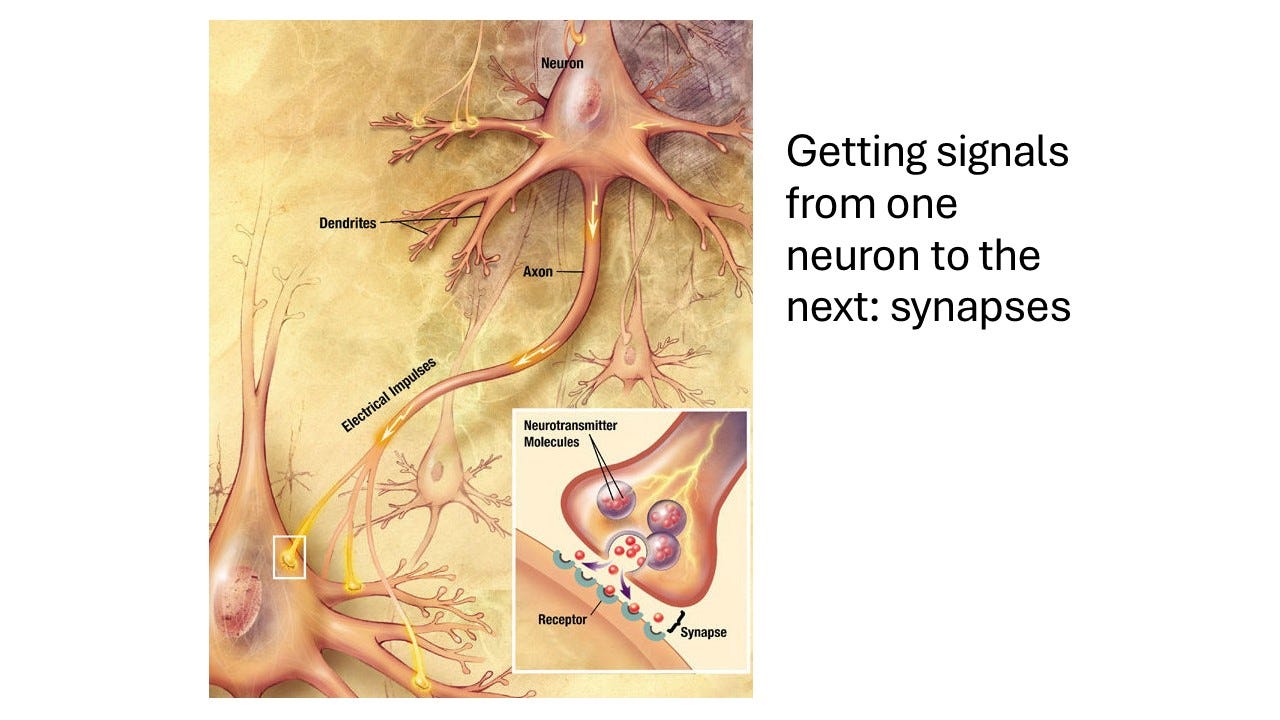
If you imagine a neuron in the brain, this neuron, through its axon, connects to thousands of other neurons. There will be currents flowing in through the dendrites into the soma. Here you've got these voltage-gated channels that can fire off action potentials. The action potential will then make the neuron fire its impulse through the axon. And then the impulse arrives on the next neuron. Something needs to happen to make that next neuron do something. The points of contact between two neurons are known as synapses. It is really just Greek for touching together. This is the point where two neurons touch each other. The finger of it, so to speak, is the presynaptic part. And then the part that is being touched is the so-called postsynaptic part. And in between them is a very small gap, the synaptic cleft.
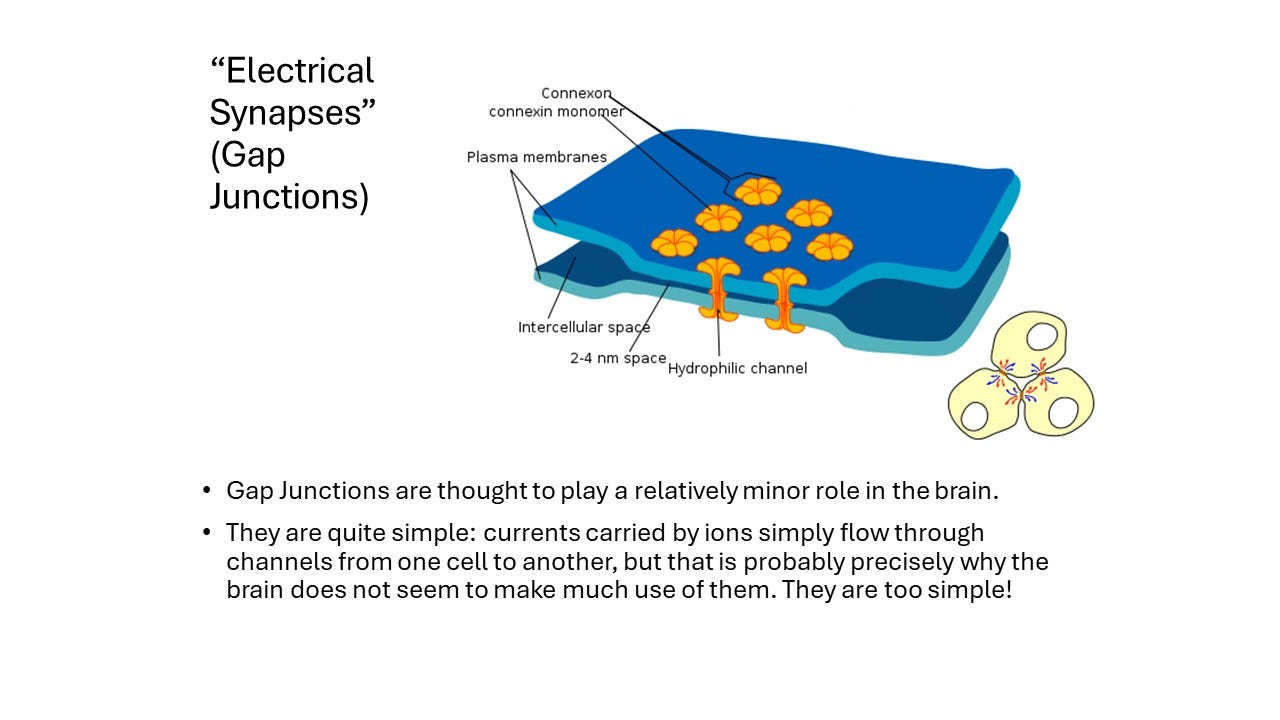
Now synapses come basically in two flavors. One uninteresting flavor is electrical synapses. The interesting one is chemical synapses.
Electrical Synapses
How do the electrical synapses work? If you want a current, basically what you want is currents flowing down the axon and you want those currents to do something in the next neuron. Well, what you could just have little pores where the ions could just be pushed through to set up a current in the next neuron, and depolarize the membrane there. Occasionally, neurons do this, but they don't do it much. In the central nervous system of mammals, very, very little stuff is done with these electrical synapses. They have a beautiful simplicity, but they have very little power compared to a chemical synapse, which is much more complicated, but because it's more complicated, it can do a lot more stuff. So the chemical synapses are really where it's at. And all the rest of the post is going to be about chemical synapses.
Chemical Synapses
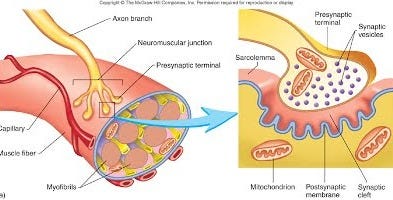
We can also introduce a very particular type of synapse, which isn't in your brain, but you have them all over your bodies, that's the neuromuscular junction synapses.
If your nervous system wants you to move, it's also going to tell your muscles, it's time to move. And that too is done actually with chemical synapses. And these chemical synapses, known as the neuromuscular junctions, are in many ways like the synapses in the brain, only that they're much larger, and they're much easier to get to, so people have studied those first. And a lot of what we know about chemical synapses in the brain was first worked out with the neuromuscular junctions.
And there's still a lot that's interesting about neuromuscular junctions, even if you're mostly interested in how the brain works. So basically these are muscle fibers, and they are contacted by these neuromuscular junctions, and your action potential is traveling down and every time action potential arrives at the synapse, the muscle fiber that would get activated would sort of twitch. And you can make them twitch very fast to get a constant contraction. And you can do that to many muscle fibers if you want a strong contraction, or just a few of them if you want a weak one, and so on. This is basically how the system operates. But what you basically see, if you were to look at them, you see what people call the synaptic bouton, the presynaptic thing.
You have the axon and the membrane bulging out into a little presynaptic bouton. Bouton is just posh for button, because it looks button-like. And this presynaptic bouton has in it tiny little vesicles. These little vesicles are made out of the same sort of phospholipid membrane as the outside. And because they are made of the same sort of membrane, they can fuse with that membrane, as if you had one bubble merging with another bubble. But of course if they fuse, then whatever is inside them can be released into the space here that abuts the next neuron. So basically you have these vesicles which are filled with special substances known as neurotransmitters.
Neuron communication via neurotransmitters
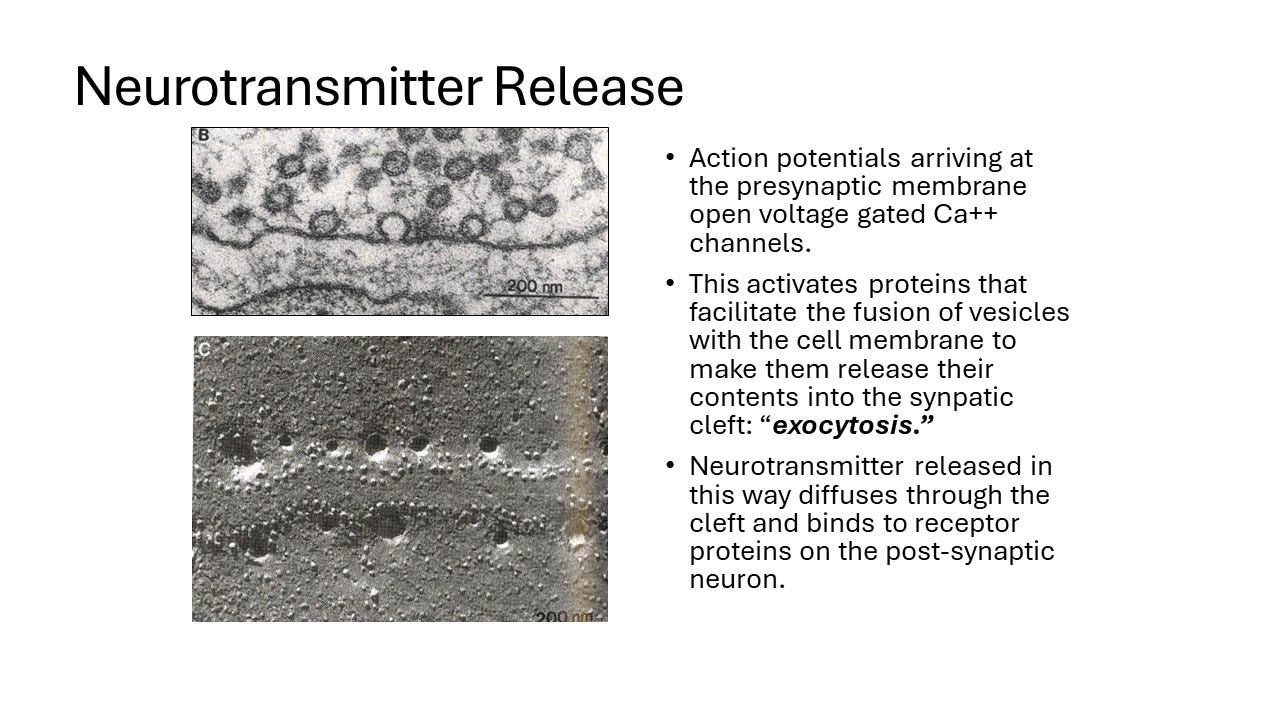
When an action potential, or a nerve impulse, arrives, the membrane will become depolarized. And there are little channels that are voltage-gated, just like action potential channels, but they're special action potential channels in that they let calcium in, rather than sodium. Most of the voltage-gated channels that carry action potentials are the sodium channels, but these ones are calcium channels. So then suddenly you get calcium rushing in here. Now calcium normally is very low in cells, so if you've got calcium suddenly rushing in, it provides a very powerful signal, and this signal activates a lot of little proteins that are stuck here, which will basically grab one of those bubbles and make it fuse. One of these vesicles will make it fuse with the outer membrane, and shed the neurotransmitter into this synapse. And then on the other side of the next neuron along, then you just get passive diffusion.
As we discussed molecules are bumping into each other and are churning and churning. The gap is nanometers wide, it's very small. The speeds at which molecules bump into each other are very high, hundreds of kilometers, so even though it is a random walk for these neurotransmitter molecules to arrive at their receptors, it does not take very long. It is going to take a small fraction of a millisecond until the neurotransmitter molecules arrive at the receptors.
The postsynaptic density, which is really basically the membrane of the next cell, has special proteins on it.
You can see the presynaptic bouton, and you can see lots and lots of vesicles, all brimming full of neurotransmitter waiting to be released when the next action potential comes along. And then you have the postsynaptic membrane, which is kind of fuzzy because it's full of little protein receptor molecules that are sitting on it, and they're just waiting for the neurotransmitter to arrive. And we know that this sort of fusion actually happens because people were able to observe it, though, not real time.
We are looking at things that are terribly small, maybe 200 nanometers. So that's a fifth of a billionth of a meter. Just to put it in perspective, we're really looking at very, very small things. They're basically smaller than the wavelength of visible light. That's why you can no longer see them with an ordinary microscope. You have to look at it with an electron microscope. Electron microscopes are sort of complicated things because you have to make your optics with electron beams. Electron beams don't travel very well through air, so you've got to put it into a vacuum. You can't do this with live tissue, so to get pictures, what people actually did was to take an isolated frog leg, put it over a bath of frozen nitrogen, dip it in, let it freeze completely and get a picture of the neuromuscular junctions.
Now that means it's not alive, but it's not dead either. And what you can do is actually stimulate these nerves in a frog leg, and still get little twitches of muscles in the frozen state. It's incredible how it works. So if you know that when you're giving it a stimulation, and in a millisecond you will have a signal, you can actually take these muscles out, put them into a bath, and then see them. Then you can actually catch these events as they occur. You can freeze-fracture them, and actually see these vesicles fusing. And you can see the postsynaptic stuff, and so on. So even though we're never quite observing this live, we've got very good reasons to believe that this is exactly how neurotransmitter release works, by basically these bubbles fusing when the calcium arrives.
The receptors
Now let's say a few words about the actual receptors. One of the typical receptors is the acetylcholine receptor. Acetylcholine is a neurotransmitter that's used for communication between nerve and muscle cells, but also a little bit in the brain. But the ones that are used most of the time in the brain are glutamate, which is the most common excitatory neurotransmitter in the brain, and GABA, which is the most common inhibitory neurotransmitter. And they both have their own specific receptors. But fundamentally they work on the same principles.
So you have your receptor, which is a protein, and it's made out of chains of amino acids. And these chains of amino acids are hydrophilic in some cases, which means they will dissolve in water, and they are hydrophobic in other places. That means they don't like water, they like oil. And that means that you have oil, they can sit quite comfortably in the membrane.
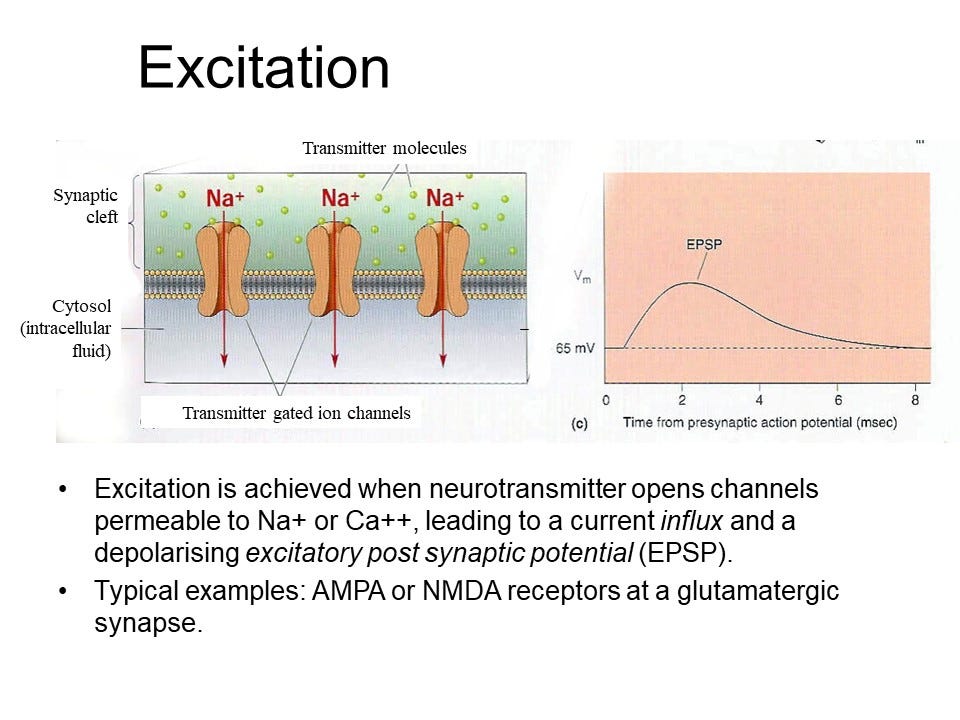
If these proteins form together, they can create a channel in the membrane. When acetylcholine binds to a pocket on the receptor, it opens a pore wide enough for sodium ions to enter the postsynaptic cell, causing depolarization and excitation. The acetylcholine receptor isn't highly selective; it allows sodium and potassium ions to pass through. However, since the cell is already permeable to potassium, the additional sodium permeability causes more sodium to enter than potassium to exit, leading to depolarization. Just adding a little bit more potassium doesn't really do much. But suddenly making the cell permeable to sodium does a lot, because sodium is normally kept out of the cell. So you get a lot of sodium flowing in, so the main thing that happens is that sodium flows in. So basically it's a sodium channel.
But of course you do get a bit of other ions flowing in, because these neurotransmitter receptors are not very selective. It is the leakage channels, or the resting channels, or voltage-gated channels that are very, very specific to certain ions. But you still have a little bit of specificity in here. So these ones for acetylcholine, aren't really allowing calcium to go through.
Cleaning up after excitation
If we are exciting the post-synaptic cell, which might be a muscle cell, and send an action potential down to a muscle, the neurotransmitter arrives, it depolarizes my muscle cells, the muscle cells get excited, and they twitch. This is great, but you want that to stop, because once you say I want to raise my arm, there comes a point when you like to lower it again. So you have to have something that makes these things close again. So, the acetylcholine which has slotted itself into this keyhole, doesn't stay there forever.
Acetylcholine is a very small molecule, relatively speaking, we have lots of water molecules bouncing around, they will just knock it out again. And off it will go, it will diffuse, and this will close again all by itself, but of course, if the acetylcholine stays around, it can just go back in. And so you need to have something that mops up the neurotransmitters after the synapse has been activated. So all synapses have some mechanism that mops up neurotransmitters. In the neuromuscular junction, you have an enzyme sitting here that's called acetylcholinesterase.
So basically, if any of the acetylcholine molecules bumps into the acetylcholinesterase, it's going to take that acetylcholinesterase, it's going to crunch it in half, and turn it into acetic acid, which is the same stuff that makes vinegar sour, and choline, which is the substance that you also find in egg yolks. And the acetic acid just sort of wanders off, the choline gets actually pumped back into the presynaptic terminal to be recycled, then there's another enzyme, cholineacetyltransferase, which puts another acetyl group onto it to make new acetylcholin, which then gets packaged in another vesicle, ready for the next impulse.
So we have an enzymatic mechanisms, but not all neurotransmitters have enzymes here that break down the neurotransmitters, sometimes they just simply have pumps, they will just pump it back in. So you squirt it out, you let it do its thing, and then you sort of suck it back in. All of this of course has to happen in just a millisecond or so, the whole thing is very, very quick. And it can be so quick because it all happens on tiny, tiny scales, where the distances that have to be travelled are very small, and the velocities with which these molecules can sort of move about are very high.
As we have already mentioned, there are lots of different types of neurotransmitter substances, and they all work more or less in the same way.
We don't even know yet completely how many neurotransmitters there are. It's actually quite surprising that only the last five, six years or so that people became aware of the fact that there is a substance known as anandamine in the brain, which is actually a neurotransmitter. People had always, for a long time, been clear about how cannabis works. But only recently people have actually discovered that there are receptors in the brain for a substance known as anandamine, and cannabis works by binding to the receptors of that neurotransmitter.
Neurotransmitters diversity
So there are still neurotransmitters that have been recently discovered, but we know a lot of them already, and we certainly know that some are used a great deal more than others. And as we mentioned already earlier, by far the most common that are used in your brain are glutamate and gamma-aminobutyric acid, or GABA for short. Now glutamate, of course, you may also remember from a couple of posts ago, is an amino acid. So it's a building block of proteins.
So glutamate, you just simply get by gobbling protein-rich food and people use it as a flavor enhancer in Chinese food. And there's often people say, “oh look, if I eat Chinese food, I get terrible neurological reactions”. Well, actually, the brain is very well shielded from dietary glutamine, and from dietary glutamate going straight into your synaptic spaces. The blood-brain barrier will keep the glutamate out.
So there's really no reason to assume that having glutamate in your food would make you sick. Now while there are several neurotransmitters, a neuron will usually only use one. So if one neuron releases glutamate in one vesicle at one synapse, then you can be pretty sure that it will use the same neurotransmitter for every vesicle for all of its synapses.
And that means that you can actually classify neurons by what type of neurotransmitter you use. Sometimes people speak of glutamatergic neurons. That means this is a neuron that operates by releasing the neurotransmitter glutamate. Or you can have a glycinergic neuron, which releases glycine.
Or a gabaergic one, or a norepinephrinegic one, or a serotonergic one, and the list goes on and on and on. So we will be using these terms later. We will talk about dopaminergic neurons and serotonergic neurons, and what particular roles they might have and what that means also for psychopharmacology.
So lots of different types of chemical transmitters. Chemically they come in different classes. Some are amino acids, which we find in foods, like glutamate of glycine.
Glutamate we already mentioned. Glycine is also just an amino acid that you can get out of digesting protein. Other amines are synthesized by special enzymes, such as the catecholamines.
Let’s talk about noradrenaline, also known as norepinephrine. This is just simply American usage versus British usage. The Americans more often use the word norepinephrine. The Brits Europeans use noradrenaline more.
It's called like that because you've got adrenum in here, which means that which is by the kidney. Why is that? Because this substance is actually very similar to adrenaline, which is a hormone that's released by the adrenal gland, which sits next to your kidneys. And of course, we all know the idea of having an adrenaline buzz.
So you can actually have hormones operating in ways that are very similar to the way that neurotransmitters operate, only that the hormones will be coursing through your bloodstream while the neurotransmitters travel nanometers before they do their job. So the neurotransmitter will do something very, very local, whereas an enzyme, or a hormone, will just go all over the place. And noradrenaline is very similar to adrenaline chemically.
Dopamine. Another very important catecholamine. We'll say more about that later. These are made from the amino acid tyrosine, which you find in your food.
Another favorite one, also more commonly known as serotonin, synthesized from the amino acid tryptophan, which you find in such delicious things as broccoli.
Now, if you don't have enough serotonin in your brain, some research suggests that you may be in danger of developing clinical depression, though it's not relly as simple as that.
Then there are peptide neurotransmitters.
Neurotransmitters are just keys, but they can open different doors
Now the thing is there's so many different neurotransmitters. And one thing that's kind of a bit pervasive in the popular literature is the idea that you have a neurotransmitter which is a neurotransmitter for something.
I've just mentioned the fact that if your serotonin deplete, the mood may go down. So you can kind of think, oh, therefore serotonin is the good mood neurotransmitter.
And your brain releases it in order to send the signal, I'm in a good mood.
That is a very, very naive and silly way of thinking about what neurotransmitters do. Because, if you think of them like little keys that are being released and there are little doors that they open, what matters is what's behind the door, not what the key is.
And because there are a couple of dozen different neurotransmitter substances, but there are a hundred billion neurons, an awful lot of these neurotransmitters are serving a lot of different functions and different neurons that do very different things. So glutamate that might be released in a particular part of my retina, for example, will say there's a flash of light over here. The very same glutamate released in a little synapse in my ear will say there's a sound of a particular frequency. And the very same glutamate that's released in my forebrain may say, remember to pick up the kids when you go home from work. So a neurotransmitter signals depends on where it is being released and by which neuron and at which time and in which context and so on and so forth.
And to just basically say, blanket, okay, neurotransmitter X is for Y is silly.
Neurotransmitter X is for diffusing across this cleft and to do something to the neuron behind. But neurotransmitters can basically do a number of different things and they can do things also that electrical synapses can't do.
Neurons can be excited or inhibited by chemical synapses (not electric synapses)
The thing that we have described is that we have a transmitter molecule that binds to the receptors that allows positively charged ions to flow in like sodium and then you get a depolarization.
And this current that is carried by these positively charged ions that will flow in is called excitatory postsynaptic current or EPSC for short. It will depolarize the cell so you can make it less negative.
But you can have different receptors that instead of letting sodium ions in, let chloride ions in.
So sodium and chloride were both very high outside. Chloride will have a chemical gradient that will allow the chloride to diffuse in once you've got a chloride channel that's open. And then instead of having positive charges flowing into the cell, you've got negative charges flowing into the cell. Now negative charges flowing in is electrically equivalent to a current flowing out.
And that will make the cell membrane more negative, not less negative. Now being less negative is exciting for a neuron.
So if they become more negative, they become inhibited. So you can have chemical silence producing inhibitory postsynaptic potentials and create an inhibitory postsynaptic current simply by being permeable to chloride rather than being permeable to sodium. And that is a trick that's very useful for the brain for a variety of reasons.
And it's something that electrical synapses cannot do because if you have just a pore, then you can just push excitation flowing through it and that's the end of it.
With chemical synapses you can have glutamatergic synapses that have sodium channels associated with them, which allow excitation of the next neuron along in the chain, or GABAergic synapses that will allow chloride to flow in, in order to inhibit the next neuron along.
So the next neuron along will therefore be have some synapses converging onto it, some of which make it excited, others of which make it inhibited.
And then they themselves become either more or less excited and thereby more or less likely to fire action potentials themselves.
Neurons will typically be either glutamatergic or GABAergic.
So, we mentioned that there are many different types of neurotransmitters. But what makes it even more complicated is that each one of those different neurotransmitters can actually act on a number of different receptors.
Typically, as we mentioned, a molecule of GABA will go to a channel that allows chloride in and just open that channel. But there may be other receptors that work differently and do different things. These type of receptors that are themselves ion channels, they are just gates that you simply open and they let an ion through, are known as ionotropic receptors. Ionotropic comes from the Greek tropane which means to take a path. Tropos, the path. They basically allow a path for the ions.
Metabotropic receptors
Now, there are other types of receptors which are metabotropic. They don't provide a path for the ions, they provide a path for the metabolism.
They do much more complicated things. And a single synapse can have both ionotropic and metabotropic synapses side by side.
So what do we mean by metabotropic?
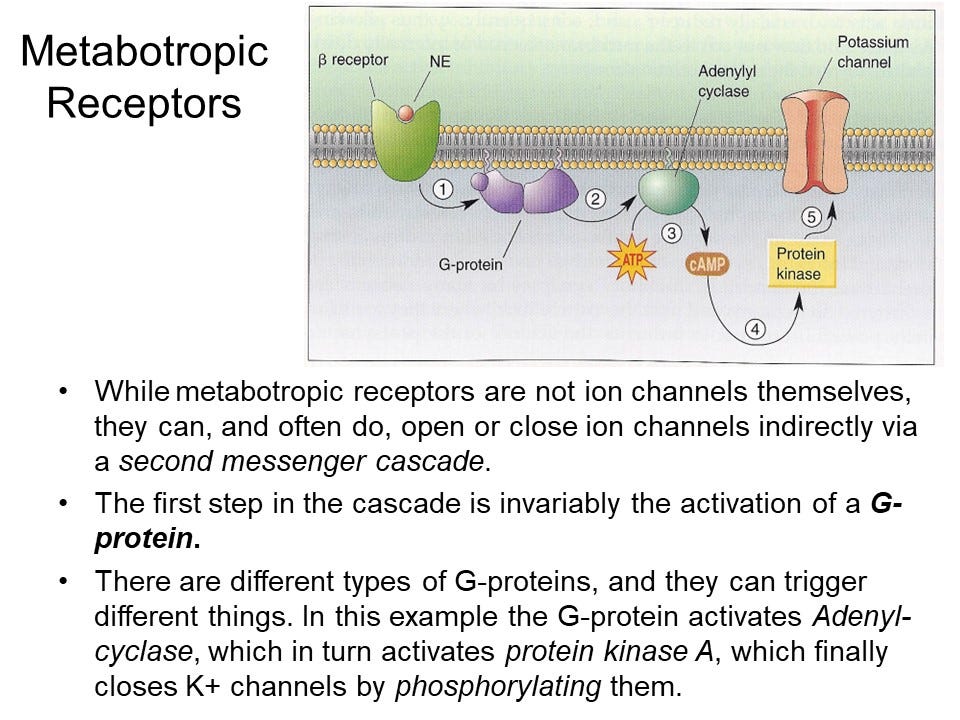
The beta-adrenergic receptor doesn't allow ions to pass through because it doesn't have a pore like ion channels do. Instead, when it binds to norepinephrine, it activates a G-protein located inside the cell near the membrane. G-proteins are composed of three subunits: alpha (α), beta (β), and gamma (γ). Together, they form a heterotrimeric G-protein in its inactive state. The receptor signals the G-protein, which causes the G-protein to split into two parts: an alpha subunit and a beta-gamma complex.
One part of the activated G-protein, usually the alpha subunit, then interacts with an enzyme called adenylyl cyclase. This enzyme converts ATP (adenosine triphosphate) into cyclic AMP (cAMP), a second messenger.
Cyclic AMP then activates another protein called protein kinase A (PKA), which is an enzyme. Once activated, PKA can phosphorylate various target proteins, meaning it adds a phosphate group to specific proteins, including potassium channels. Phosphorylation means attaching a phosphate group to a protein, which can change the protein’s function.
In this case, phosphorylating the potassium channel alters its activity, causing it to close. As a result, potassium ions can no longer flow through the channel, affecting cellular function.
Consider potassium leakage channel. Normally, these channels help maintain the cell's resting membrane potential by allowing potassium ions to flow out of the cell, keeping it polarized at a low resting potential. But when some of these channels close, the cell's membrane potential becomes less negative, or depolarized.
As a result, the cell's resting potential rises, making the cell more excitable. In other words, the cell is now closer to the threshold needed to fire an action potential. This whole process makes the cell more responsive to stimuli.
But this seems like a complicated way of achieving that effect. You could imagine that a simpler solution—like just letting sodium ions flow into the cell—would also depolarize it and increase its excitability. So why use such a slow and complex mechanism involving multiple proteins?
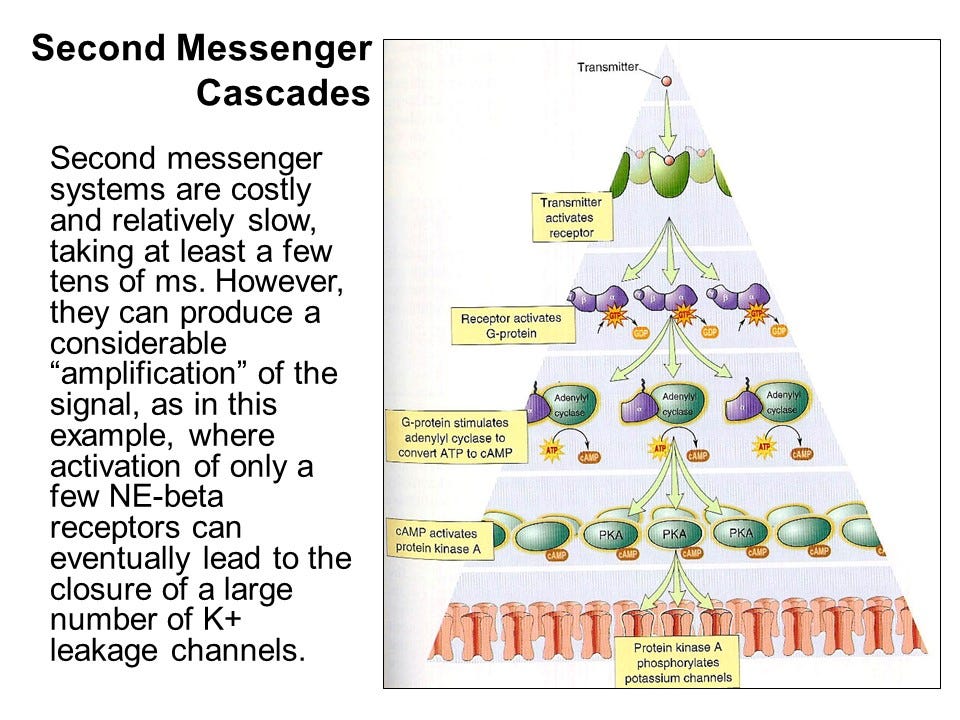
Well, there are advantages to this complexity. One key benefit is signal amplification. A single beta-adrenergic receptor doesn’t just interact with one G-protein. When norepinephrine binds to the receptor, it can activate multiple G-proteins sequentially. Each G-protein can then activate adenylyl cyclase, which produces many molecules of cyclic AMP.
This amplification means that one activated receptor can result in the production of thousands of cyclic AMP molecules, which can lead to the closure of hundreds of potassium channels. So, while the process is slow and complex, it allows for a massive amplification of the original signal, creating a large cellular response from just a single binding event.
A small amount of a neurotransmitter or hormone can have a huge impact on a cell. This happens because of signal amplification. These amplifications are part of what are called second messenger cascades. Essentially, when just one transmitter molecule binds to a receptor, it can activate many G-proteins, which then go on to activate multiple enzymes like kinases and cyclases, creating a large chain reaction. This is why substances like adrenaline are so powerful.
For example, when even a tiny amount of adrenaline is injected into a person, their heart rate rapidly increases, and their blood pressure rises, all from a very small initial stimulus. The signal is massively amplified within the cell.
Metbotropic receptors and gene transcription
Another advantage of metabotropic receptors (like the beta-adrenergic receptor) compared to ionotropic receptors is that metabotropic receptors can influence gene transcription. The second messenger cascades triggered by metabotropic receptors can interact with proteins involved in reading the cell’s genome.
The genome contains the instructions for making all the proteins the cell needs. By influencing which proteins are produced, the cell can change its function and behaviour. This allows metabotropic receptors to bring about long-lasting changes in the cell, with effects that can last for hours or even days, whereas ionotropic receptors typically have faster but shorter-lived effects, lasting only milliseconds.
This is why it's beneficial for cells to have both metabotropic and ionotropic receptors—each type plays a unique and important role in cellular communication.
Final look at neurotransmitters
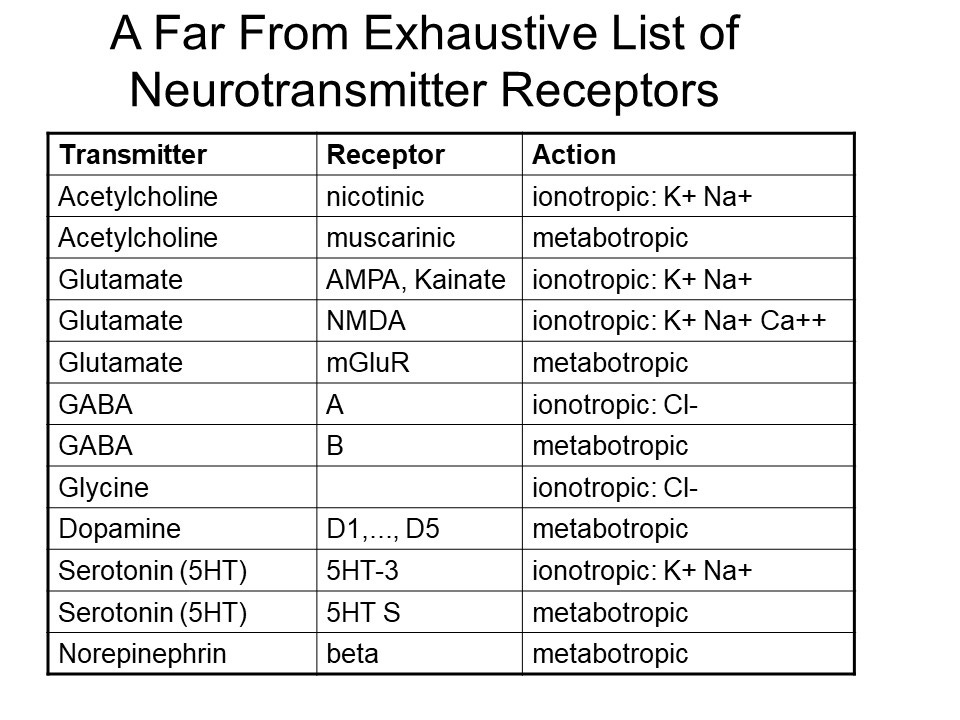
Let's take a quick look at a few common neurotransmitters. This is just a brief list but it's helpful to be familiar with these. Some common neurotransmitters include acetylcholine, glutamate, GABA, glycine, dopamine, and serotonin.
For acetylcholine, there are two main types of receptors: nicotinic receptors and muscarinic receptors. Nicotinic receptors are called that because they can be activated by nicotine. So, if you smoke a cigarette, these receptors respond.
Nicotinic receptors are ionotropic, meaning they allow ions like potassium and sodium to pass through, exciting the cell. This ionotropic mechanism is similar to what we see at the neuromuscular junction, where acetylcholine triggers muscle contraction.
If you were to take a large dose of nicotine—say, if you injected it or, in an extreme case, consumed a large quantity—you would overload your acetylcholine synapses, causing them to fire excessively. This could lead to widespread muscle contractions throughout your body, which would be extremely dangerous.
The tobacco plant produces nicotine as a defense mechanism. If an animal, like a cow, eats the plant, it will likely avoid it in the future due to the toxic effects. However, humans, in a different context, use nicotine to affect the brain.
There are also muscarinic receptors, which are metabotropic and have more complex effects when they interact with acetylcholine. The name "muscarinic" comes from Amanita muscaria, a type of poisonous mushroom.
When it comes to glutamate, the main excitatory neurotransmitter in the brain, there are several types of receptors. These include AMPA receptors and kainate receptors—both of which are ionotropic and allow ions to pass through. Another important glutamate receptor is the NMDA receptor, which is also ionotropic but has different properties, including allowing calcium ions to enter the cell.
Chemists have developed selective agonists—chemical substances like AMPA or NMDA—that can specifically activate one type of glutamate receptor but not others. There is also the mGluR receptor, which is metabotropic, meaning it works through second messengers rather than ion flow. Each of these receptors plays a different role in neural signaling.
As for GABA, it's also a key neurotransmitter, but in contrast to glutamate, it’s inhibitory. However, glutamate is crucial for most brain functions, particularly for thinking and learning. In fact, in most cases when we talk about synapses without specifying the neurotransmitter, there's a 90% chance that it's glutamate.
GABA, the most important inhibitory neurotransmitter, has GABA-A receptors which are ionotropic and GABA-B receptors that are metabotropic. These tend to be inhibitory in their action.
If you enjoy a glass of brandy and then you drink another, and then you drink another, and then you start getting a little bit tired, that's because these GABA receptors actually can get activated by high doses of ethanol, alcohol. And they are inhibitory, so they will start to make you doze off.
There are a lot of sleeping pills that were developed to activate GABA receptors. They will just simply up the inhibitory in neurotransmission in your brain, and thereby they don't really make you go to sleep but they will anesthetize you. So you'll sit there still and quiet and think you're sleeping but it is not as good as a proper night's sleep.
Another important inhibitory neurotransmitter is glycine, which works through ionotropic chloride channels. As for dopamine, there are five different types of receptors, all of which are metabotropic. Serotonin has many receptor types as well, with some being ionotropic and others metabotropic.
This is a long list, and pharmacologists are very interested in these receptors because they aim to develop drugs that target specific ones. For example, they might create a drug that activates a certain receptor to help with sleep, treat erectile dysfunction, cure depression, or manage addiction. Many drugs that affect these receptors can either cause addiction or help people overcome it by altering the chemical signals between neurons.
Interestingly, coffee works differently from many other substances. Unlike drugs like cocaine, which directly activate neurotransmitter systems (such as the dopamine pathway), coffee does not directly affect neurotransmitter release. Instead, it acts on adenosine receptors, indirectly impacting how awake and alert we feel.
But we will continue to discuss neurotransmitters in the next post.