Post #3 of this series on the Brain (First Post and Previous Post). As I mention every time, these posts are from a series of lectures by Prof. Jan Schnupp, and I want to make sure he is properly quoted and credited. Many parts are his lectures verbatim. However, for any errors, mistakes or inaccuracies in anything I write in here, I take all the credit. I probably misunderstood him or made it up. His course material is available here.
So, last time we have zoomed right into neurons and basic chemistry. We discussed that there is a fluid within the cells and a fluid around the cells, the neurons, made out of dissolved salt in water.
You also have to imagine, of course, that there is a lot of movement going on.
These water molecules would actually be pushing around and being moved around at rapid speed. And the reason for this is relatively simple: it is heat. If you have a substance that is either liquid or a gas, then it is liquid or gaseous because all the molecules are constantly moving and do not stay in their place. And the hotter the substance gets, the faster they move. And this leads to constant mixing about.
The molecules bounce into each other and, even though they started off each in just a particular place, they end up distributed all over the place. This is known as diffusion. So if you put sugar in a tea and you leave the sugar lump at the bottom, initially all the sugar is just going to be at the bottom. But if you come back two hours later, the sugar is going to be all over. It is going to be all through the tea. The hotter the tea is, the quicker you are going to get it mixed all over.
So for the individual molecules, the smallest molecules may actually be bouncing around at average velocities in the order of hundreds of kilometres an hour. On the very smallest scale, your brain or any part of your body actually is a very violent place.
We have water molecules bashing into each other 100 kilometres an hour. But the other thing that I would like to point out to you here is that, on average, no two of the molecules move at the same speed. Why would they? They bash into other things that may accelerate them or slow them down. But actually the smaller molecules tend to move on average much faster than the large ones, which is perhaps not surprising.
The big heavy ones, if you imagine a truck and a bicycle colliding, the impact on the bicycle is going to be much larger than on the truck. So if you have very small molecules bashing at very high speed into a very, very large molecule, it is not going to make a huge dent on the large molecule, but it is going to make a big dent on the small molecule. The other thing that I would like you to take away from this diffusion, this tendency for there to be just random motion, which comes about by heat, is that it is perhaps the most important driving force for all activity in your brain. Your brain is powered, and this is also kind of a weird thought, by random noisy churning. Water churning in your brain cells, and we are going to talk about this churning, and salt ions moving around and also bumping into other molecules.
And of course, the fact that this sort of randomness is one of the key things that make brains operate, I think is actually kind of interesting because we tend to think of ourselves as rational, and if you calculate two plus two in your head over and over and over again, you will always come up with the answer four. But of course, that is not really what our brains are about, is it? I mean, if there is a lot of randomness going on in your brain, maybe that is entirely natural. And of course, you can make it all less natural by cooling it down. But what is going to happen to a brain if you cool it down? Well, what happens if you have a bruise and it hurts and you put an ice bag on it? It makes it numb, doesn't it? The reason that it makes it numb is because you are going to slow down all the molecules in the nerve cells on your skin, and as soon as you cool them down, as soon as you make them just a little bit less fast, less bounced into, they can no longer do what they normally do.
So cold is actually a very powerful anesthetic because if you just cool the nervous system down far enough, it will stop functioning. It needs this sort of constant motion of heat. So we have now got our water, which is full of electrically charged ions bouncing this way and thereby producing electrical currents that go this way and that way. But of course, if you have positively charged ions going one way and then negatively charged ions going the other way, on average, it is all going to cancel out. You are not really going to get an electrical current that can carry a signal in a systematic way. We somehow need to get some order into this. We need to make sure that any electrical currents that we get by ions that are bouncing around in this water need to be channeled in a sensible way. The way nerve cells do this is by using fat. The thing about fat is that it does not mix with water. So why doesn't fat mix with water? Well, fat is basically made out of hydrocarbon, so we have long molecules, typically in the order of 12 to 20 carbon atoms, each with lots of little hydrogen atoms forming long chains.
These fatty acid molecules, as they are known, these sort of long chains of carbon atoms with hydrogen on them, unlike the water, will distribute their electrons very fairly and very evenly among them, so they are not polar. So this mutual attraction that one water molecule has to another water molecule, they do not share with fat. They become kind of uninterested in the fat. Most fats in the body will be made out of chains of so-called fatty acids, where you have basically got lots of carbon atoms lined up. And then on the top, you have got a so-called carboxy group.
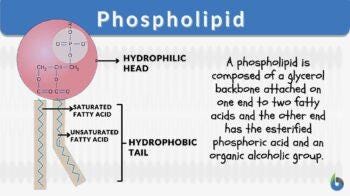
So the fat that we all have will actually be made out of three fatty acid chains linked together on a glycerin at the top.
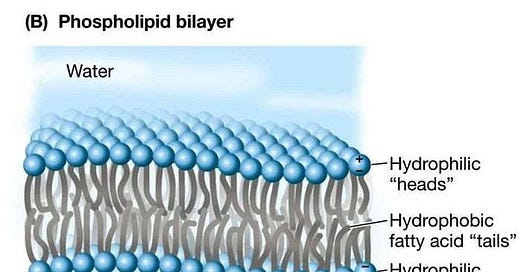
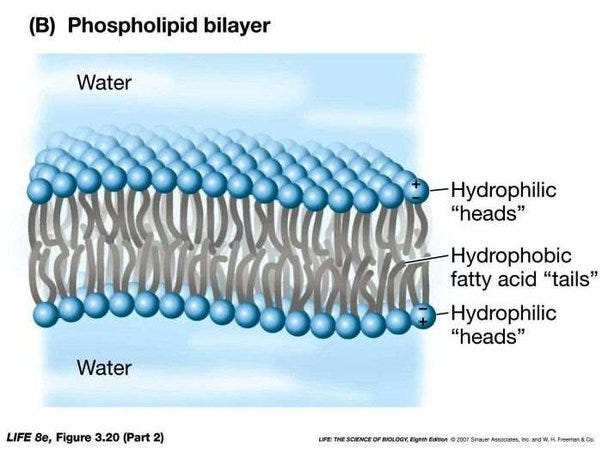
But the fat that we are particularly interested from the point of view of understanding what neurons do are so-called phospholipids. So the idea is you have two fatty acids, and then you have got a phosphate group here with a little choline at the top.
So what does that do? Well, this phosphate actually itself is an iron. It is negatively charged because it has taken on an extra electron, one more than the number of protons in it. And then you have a choline at the other, which is also an iron, in this case a positively charged iron. So we havet stuff here that has very strong electric attraction to water molecules. And then we have bits that have no interest for water molecules whatsoever.
So we have a so-called hydrophobic water-fearing or water-repellent part to this molecule, and we have a water-attracting part to this molecule. And if I just have enough of these molecules, these phospholipids, and I put them in water, they will automatically line themselves up to form a phospholipid bilayer, where they basically just put all the water-attracting head regions out towards the water, and all the ones that are the water-repellent parts just line up against each other. So I end up with just a thin layer of fat. And this thin layer of fat, which is really only two molecules thick, is the lining of all the cells in your body. They are all lined with these phospholipids. Which is why, no matter how diet-obsessed you may or may not be, you need some fat in your body. You need to be able to line every cell in your body with these lipids, or else your body will break down.
Because it needs that in order to be able to control the flow of liquid and the flow of ions, dissolved salts, around your body.
Because this now provides a barrier. Bear in mind here we have got our water molecules here bashing around. But these molecules are pretty big, and they are stuck together in this sort of conformation that is actually pretty solid. So if the water molecules bounce into this, they will just bounce back.
And any ions that they carry with them in this hydration shell, similarly, will not be able to push through here. So, suddenly, I have a material that allows me to channel the flow that ions can take in my brain. I can send it down there. I can basically make big tubes out of this, which forms little cables through which I can send the electric current.
And it is really tubes like this which are the axons and the dendrites of the neurons that we have seen earlier. So we have basically got these neurites, as they are collectively known, which are tubes made out of these phospholipid bilayers. And we can have ions on one side, and different ions on the other side.
There is one thing to just quickly mention before I introduce another important layer, which is the proteins. I told you about salt forming ions and how they get dissolved (previous post). And of course, for salt, we normally think sodium chloride. And sodium chloride is perhaps the most important salt in neuroscience, but potassium is just as important.
Potassium salts and calcium salts are also very, very important.
So you can basically get so-called cations, which are positively charged ions. Sodium, potassium, calcium are the ones that we are going to hear more about. And then anions, sort of negatively charged ions.
We have got chloride, and we have got so-called organic anions.
What does organic mean? What organic literally means is that it is made out of hydrocarbons. The word organic already had a meaning for hundreds of years, which means hydrocarbon chemistry, effectively.
So we will introduce some organic anions next. Because the organic anions that we are interested in here are so-called proteins.